American-made Isolators designed to
exceed industry standards.
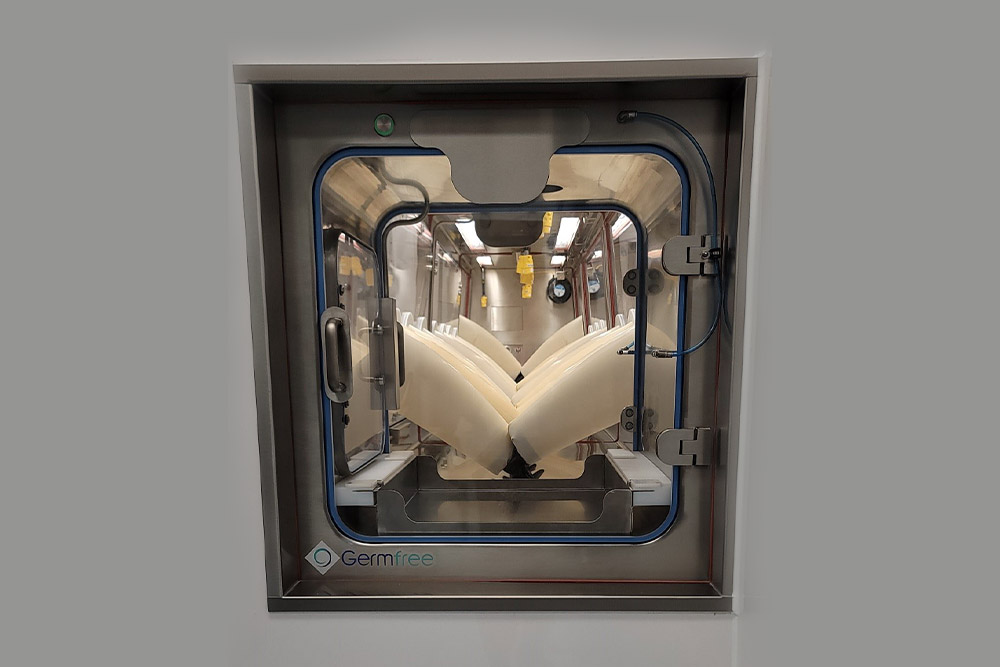
In pharmaceutical manufacturing, every step matters when it comes to protecting patients and ensuring product quality. Germfree designs pharmaceutical isolators that not only meet strict industry standards but also give manufacturers confidence in their processes. Our isolators, designed and manufactured in the USA, create a fully controlled environment where your team can work safely while maintaining the sterility of your products. Whether you’re handling highly potent compounds or complex processes, these systems are built to support your workflow and help you focus on what matters most: delivering safe, life-saving medicines.
Key Features & Benefits
Protection Type: Environment, Product and Personnel
Industries: Biopharma, 503B Pharmacies
cGMP-Compliant
- Stainless Steel Construction
- Automated Pressure Decay Testing
- Integrated EMS & Biodecontamination Systems
- Interlocking Doors
- Custom Built for Your Application
Advanced engineering for reliable contamination control.
Germfree’s pharmaceutical isolators are engineered to meet the highest cGMP standards, creating a gas-tight barrier that maintains aseptic conditions and containment. Designed to operate under positive or negative pressure, they protect sterile products, personnel, and the surrounding environment.
Suitability
Designed to meet the needs of multiple applications, Isolators are ideal for a wide range of industries and facilities.
Download the Brochure for More Information!

Pharmaceutical Isolator FAQs
1. What is a pharmaceutical isolator?
A pharmaceutical isolator is a fully enclosed, gas-tight system that creates a sterile environment for handling products. It protects both the operator and the product from contamination and includes on-board biodecontamination to keep the workspace sterile between uses.
2. How is an isolator different from an O-RABS?
Unlike an O-RABS, which relies on cleanroom conditions, an isolator is completely sealed from the external environment and typically includes automated decontamination systems, providing a higher level of sterility assurance.
3. What are pharmaceutical isolators used for?
They are commonly used in sterile compounding, handling potent compounds, and other processes where strict contamination control is critical.
4. Why choose Germfree isolators?
Germfree has been designing biocontainment and sterile equipment since the 1960s. Our isolators combine proven engineering with modern innovation, offering reliability, ease of use, and industry-leading contamination control.
5. Do Germfree isolators meet regulatory standards?
Yes, all Germfree pharmaceutical isolators are designed to comply with cGMP & EU Annex 1 requirements, as well as international regulatory recommendations.
Talk to us about your next isolator project.
Contact usContact us: Let's unlock your scientific potential together
Complete our contact form and a member of our commercial team will reach out to you within 24 hours.
Sales: + 1 (386) 265-4300
Service & support: cs@germfree.com
Send us your request